PoreStar Cranial

| ⇦ | ⇩Video information⇩ | Home⊳ |
PoreStar Patient Specific Implants
A new class of implant material
PoreStar (porous polyethylene) implants are indicated for the repair or augmentation of craniofacial bone including defects caused by trauma, tumour or a congenital disorder. This includes restoration of contour in the craniofacial skeleton and cranioplasties for the protection of intracranial structures and normalisation of cerebral haemodynamics. PoreStar surgical implants are individually designed from 3D CT scans.
PoreStar Surgical Implants
Manufactured from a star to create a unique bone-like porous architecture
Porosity may permit hard and soft tissue adhesion to secure the implant
Surgeon can perform minor modifications with a scalpel and shape or feather the edges of the implant after fixation
CT and MRI compatible; radiolucent and non-magnetic for compatibility with post-operative imaging
Supplied with a BioModel (anatomical replica) with every case
Design Your Implant
Surgeon can contribute to the implant design or decide on tumour resection margins
Online implant design review service available via AnatomicsC3D
BioModel and implant prototype can be sent to the surgeon for review with the patient as part of the design process
Porous Polyethylene - Proven Clinical History
Clinical history of reconstruction and augmentation in the maxillofacial region1
Tissue ingrowth stabilises the surgical implant2
The biocompatibility standard based on long term stability and virtual lack of inflammatory response3
| ⇦ | ➤To Surgical Product | Home⊳ |
【Video Information】
<CSIRO Anatomics create Porestar>
| ⇦ | ➤To Surgical Product | Home⊳ |

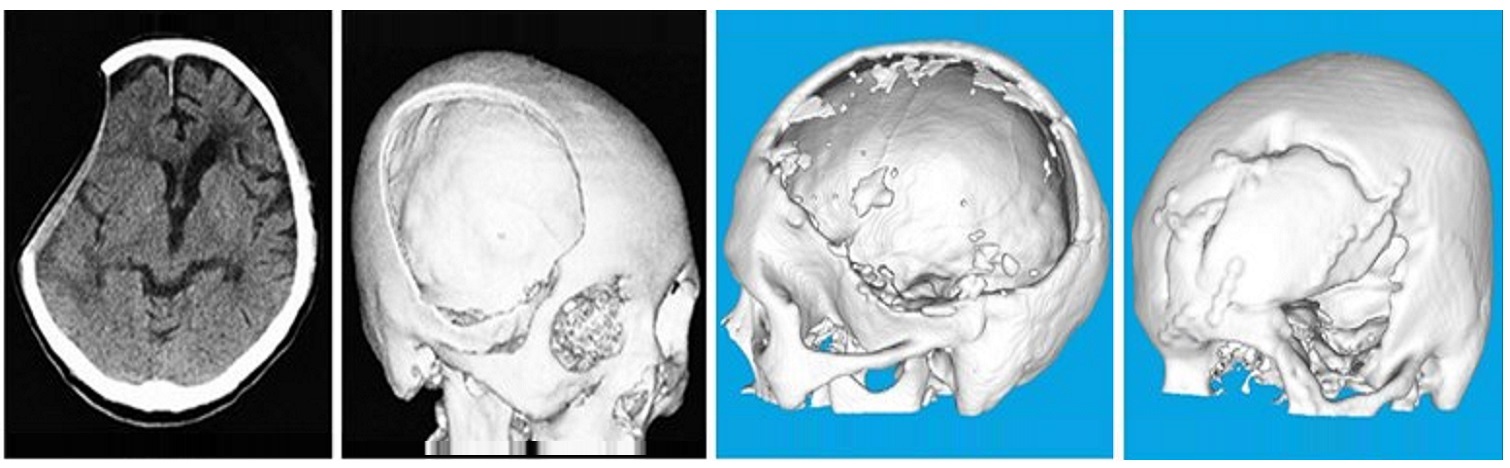 CT Scan Protocol
CT Scan Protocol